|
Papillary carcinoma |
|
Clinical presentation.
Papillary carcinomas are included in relatively fast growing nodules. The period lasting from the detection to the first visit in patients who were aware about a palpable tumor was in the range of 3-24 months with a median of 8 months in our practice. This is more fast than in the case of a benign nodule but more slowly compared with medullary carcinoma.
Palpation. As most malignant diseases, papillary carcinoma is presented generally by a firm or hard nodule. Nodules are not as hard as in the event of an anaplastic carcinoma. They are moveable, but in a less extent than benign nodules. In around 20% of cases we found enlarged, metastatic lymph nodes at the time of the diagnosis of papillary carcinoma. Palsy of the
recurrent nerve can be found in aggressive subtypes and in patients who requested evaluation only years after detection of the nodule. 10% of our patients presented with hoarseness.
Functional state. In contrast with other thyroid carcinomas, patients with papillary cancer are not infrequently subclinically hypothyroid. This is the consequence of the well-know association between lymphocytic thyroiditis and papillary carcinoma. There is a long-lasting and yet undetermined debate which is the cause and which is the consequence. Nevertheless, the concomitant thyroiditis is responsible for the usually mild and subclinical hypothyroidism. The presence of dysfunction is an interesting phenomenon but have practically no relevance in the diagnosis.
Ultrasonography.
Features with significantly increased risk of papillary carcinoma are listed in Table. From a practical point-of-view, the blurred borders of the nodule and the presence of microcalcifications are of the greatest diagnostic power.
Most papillary carcinomas occur in hypoechogenic nodules, it means more than 80% of cases. This proportion is less compared with e.g. medullary cancer or secondary thyroid carcinomas.
Irregular borders of a
hypoechogenic nodule is the most specific sign of papillary carcinoma.
Three types of irregularities have to be discussed. Ill-defined, blurred
border  is the most
important type. It means that we cannot decide where the nodule ends
and where the extranodular part of the thyroid begins. This type of
irregularity can be observed in the case of de Quervain's thyroiditis,
too. Serious differential diagnostic problems may arise in those
infrequent cases where the patient does not present typical clinical
signs of subacute thyroiditis. Naturally, in this situation FNAC is
mandatory.
is the most
important type. It means that we cannot decide where the nodule ends
and where the extranodular part of the thyroid begins. This type of
irregularity can be observed in the case of de Quervain's thyroiditis,
too. Serious differential diagnostic problems may arise in those
infrequent cases where the patient does not present typical clinical
signs of subacute thyroiditis. Naturally, in this situation FNAC is
mandatory.
 The lobulated
margins of the nodule is another type of irregularity. The
cause for this phenomenon is either the infiltrative nature of the
tumor or the proliferation of the connective tissue. In the latter a
pseudonodular appearance can be observed similarly to that seen in
certain cases of a lymphocytic thyroiditis.
The lobulated
margins of the nodule is another type of irregularity. The
cause for this phenomenon is either the infiltrative nature of the
tumor or the proliferation of the connective tissue. In the latter a
pseudonodular appearance can be observed similarly to that seen in
certain cases of a lymphocytic thyroiditis.
The sharp irregular surface of the tumor is the less sensitive and specific type of the irregularity of the borders.
Microcalcification
is the second most characteristic sign  of papillary
carcinoma. It means that small bright punctate granules can be observed
within a hypoechogenic nodule. The differential diagnostic of punctate
granules includes the so called comet tail artefact and the
intranodular fibrosis. The former granule has a small characteristic
tail. In the case of intranodular fibrosis not only hyperechogenic
granules but hyperchogenic lines can be seen depending on the angle
between the fibrotic bundle and the transducer. Not infrequently a
clear distinction between various types of hyperechogenic granules
cannot be made.
of papillary
carcinoma. It means that small bright punctate granules can be observed
within a hypoechogenic nodule. The differential diagnostic of punctate
granules includes the so called comet tail artefact and the
intranodular fibrosis. The former granule has a small characteristic
tail. In the case of intranodular fibrosis not only hyperechogenic
granules but hyperchogenic lines can be seen depending on the angle
between the fibrotic bundle and the transducer. Not infrequently a
clear distinction between various types of hyperechogenic granules
cannot be made.
Coarse
calcification occur significantly more frequently in  the case of
papillary carcinoma then in benign nodules. The practical significance
is limited. Nevertheless, in certain cases the coarse calcification may
be the only suspicious sign. It was of great help in a case where a
small hypoechogenic nodule was found in an almost identically
hypoechogenic thyroid.
the case of
papillary carcinoma then in benign nodules. The practical significance
is limited. Nevertheless, in certain cases the coarse calcification may
be the only suspicious sign. It was of great help in a case where a
small hypoechogenic nodule was found in an almost identically
hypoechogenic thyroid.
Cystic
degeneration is a very common phenomenon in  the event of
a nodular goiter irrespectively of the nature of the lesion except for
medullary and follicular carcinoma, which only rarely contain cystic
areas. On the other hand, papillary carcinomas frequently present
cystic areas. The presence of microcalcification within the solid part
of a cystic nodule increases the likelihood of malignancy.
the event of
a nodular goiter irrespectively of the nature of the lesion except for
medullary and follicular carcinoma, which only rarely contain cystic
areas. On the other hand, papillary carcinomas frequently present
cystic areas. The presence of microcalcification within the solid part
of a cystic nodule increases the likelihood of malignancy.
Although the presence of
a halo sign suggests a  significantly
decreased risk of malignancy overall, the consequences of a detailed
analysis indicate that the situation is not so simple. First, if we do
not investigate encapsulated nodules, a high proportion of cancers will
be missed. Moreover, our results demonstrate that the absence of a halo
sign is not an independent risk factor. The absence of a halo decreases
the risk of malignancy only because this sign cannot be demonstrated in
the most frequently occurring malignant nodules, in hypoechogenic ones.
The demonstration of a halo sign is very difficult or even impossible
in most hypoechogenic nodules because the US appearance of the capsule
in such lesions is identical with the nodule. This is clearly proved by
the fact, that for follicular adenomas, which are encapsulated in 100%
of the cases, only 8.8% of the hypoechogenic nodules exhibit a halo
sign, whereas this feature can be demonstrated by US in 64.1% of all
other types of nodules. It is not surprising therefore, that the
presence of a halo sign significantly increases the risk of malignancy
in cases of moderately hypoechogenic nodules, while in hyperechogenic
nodules the presence or absence of a halo sign does not influence the
likelihood of malignancy.
significantly
decreased risk of malignancy overall, the consequences of a detailed
analysis indicate that the situation is not so simple. First, if we do
not investigate encapsulated nodules, a high proportion of cancers will
be missed. Moreover, our results demonstrate that the absence of a halo
sign is not an independent risk factor. The absence of a halo decreases
the risk of malignancy only because this sign cannot be demonstrated in
the most frequently occurring malignant nodules, in hypoechogenic ones.
The demonstration of a halo sign is very difficult or even impossible
in most hypoechogenic nodules because the US appearance of the capsule
in such lesions is identical with the nodule. This is clearly proved by
the fact, that for follicular adenomas, which are encapsulated in 100%
of the cases, only 8.8% of the hypoechogenic nodules exhibit a halo
sign, whereas this feature can be demonstrated by US in 64.1% of all
other types of nodules. It is not surprising therefore, that the
presence of a halo sign significantly increases the risk of malignancy
in cases of moderately hypoechogenic nodules, while in hyperechogenic
nodules the presence or absence of a halo sign does not influence the
likelihood of malignancy.
The taller-than-wide
sign is frequently mentioned in the  literature
because it also occurs significantly more frequently among malignant
lesions than among benign ones, but proved to be of less practical
significance in compared with micocalcification or surface
irregularities.
literature
because it also occurs significantly more frequently among malignant
lesions than among benign ones, but proved to be of less practical
significance in compared with micocalcification or surface
irregularities.
The situation is similar
as regards type 3 vascular  pattern,
i.e. the presence of increased intranodular blood flow. This phenomenon
occurs significantly more frequently among papillary carcinoma than
among benign lesions but the practical value is limited.
pattern,
i.e. the presence of increased intranodular blood flow. This phenomenon
occurs significantly more frequently among papillary carcinoma than
among benign lesions but the practical value is limited.
Elastography is nowadays a very popular field in thyroidology. Nevertheless, the results gained by different authors are hard to compare because of technical reasons. Moreover, it is questionable whether in the case of a lesion with a benign appearing elastographic pattern we could avoid FNAC.
We must keep in mind, that the task of ultrasonography is not to diagnose a carcinoma, but the detect a nodule with high enough risk of malignancy to perform aspiration cytology.
Cytological diagnosis
The main features of papillary carcinoma are as follows:
- Lack of colloid
- Cellular pattern
- Papillary fragments and monolayered sheets with disturbed pattern
- Atypical cells with mild to moderate pleomorphism
- Nuclear inclusions, grooves and scratchy chromatin structure.
- Distinct cell borders
- Presence of multinucleated cells
The first 2 features are not specific for papillary carcinoma and may be lacking.
Papillary fragments are 3-dimensional figures with sharp, geometric borders. In contrast with hyperplastic papilla, they lack peripheral vacuolization and projections. There is no tendency of cells at the edge of such clusters to dissociate.
Monolayered sheets are frequently found in PC. They are characterized by nuclear crowding and overlapping and loss of polarity of cells.
Nuclear inclusions are the most important clue in the diagnosis of PC. They re
At low power
The aspirates are usually rich in cells and contain little if any
colloid. The tumor cells occur in tumor fragments or  singly, in small
clusters or in cohesive sheets. The multilayered tumor fragments, the
papillae exhibit a sharp edge, with palisading of the
cells at the periphery, and a branching structure is often seen at low
power in the microscope. The nuclear details in cells from papillary
fronds are difficult to analyze in some cases, because of nuclear
crowding. In contrast with hyperplastic benign papillary clusters,
malignant papillae lack peripheral vacuolization and projections, and
also lack the tendency of peripheral cells to dissociate.
singly, in small
clusters or in cohesive sheets. The multilayered tumor fragments, the
papillae exhibit a sharp edge, with palisading of the
cells at the periphery, and a branching structure is often seen at low
power in the microscope. The nuclear details in cells from papillary
fronds are difficult to analyze in some cases, because of nuclear
crowding. In contrast with hyperplastic benign papillary clusters,
malignant papillae lack peripheral vacuolization and projections, and
also lack the tendency of peripheral cells to dissociate.
Another type of large cell clusters may be observed less often. These monolayered
sheets resemble those seen in nodular goiters. Sheets of
nodular goiters involve evenly-distributed nuclei without overlapping,
while the edges of these sheets are irr egular. More
important is the presence of naked, pyknotic nuclei close to the
sheets. In papillary cancer, the occurrence of dispersed cells is
infrequent, and the dispersed cells have abundant cytoplasm. Moreover,
cancer cells forming clusters display the characteristic nuclear
atypia, and are not evenly distributed. The edges of malignant
monolayered sheets may also be irregular.
egular. More
important is the presence of naked, pyknotic nuclei close to the
sheets. In papillary cancer, the occurrence of dispersed cells is
infrequent, and the dispersed cells have abundant cytoplasm. Moreover,
cancer cells forming clusters display the characteristic nuclear
atypia, and are not evenly distributed. The edges of malignant
monolayered sheets may also be irregular.
 At high power
The cells of papillary cancer vary in size, but are larger than those
of nodular goiter cases. The variation in shape is also greater than in
benign cases. A great number of the nuclei are oval. This is one of the
characteristic features seen in papillary carcinoma. Small nuclei are
usually visible within the pale nuclei. The presence of large prominent
nucleoli is a rare phenomenon in the case of papillary carcinoma except
for the oxyphilic variant.
At high power
The cells of papillary cancer vary in size, but are larger than those
of nodular goiter cases. The variation in shape is also greater than in
benign cases. A great number of the nuclei are oval. This is one of the
characteristic features seen in papillary carcinoma. Small nuclei are
usually visible within the pale nuclei. The presence of large prominent
nucleoli is a rare phenomenon in the case of papillary carcinoma except
for the oxyphilic variant.
 The chromatin is
finely dispersed, giving the nuclei a ground-glass appearance, a
feature commonly seen in smears stained by the Papanicolau method, but
less frequently by Wright-Giemsa method and only very infrequently in
smears stained by the hematoxylin-eosin method.
The chromatin is
finely dispersed, giving the nuclei a ground-glass appearance, a
feature commonly seen in smears stained by the Papanicolau method, but
less frequently by Wright-Giemsa method and only very infrequently in
smears stained by the hematoxylin-eosin method.
 The nuclei not
infrequently exhibit a scratched appearance. This
sign is probably caused by the invagination of nuclear membrane as in
the case of inclusions and grooves. Another possible explanation is
that scratches correspond to condensed chromatin which is a frequent
finding in papillary carcinoma.
The nuclei not
infrequently exhibit a scratched appearance. This
sign is probably caused by the invagination of nuclear membrane as in
the case of inclusions and grooves. Another possible explanation is
that scratches correspond to condensed chromatin which is a frequent
finding in papillary carcinoma.
 A peripheral
sharp dark ring of condensed chromatin is present in most of
the cases stained by the Papanicolau method . Even more important is
the presence of nuclear grooves and inclusions
within the nuclei. Both of these are artefacts,
and reflect intranuclear cytoplasmic invagination. The precise name
would be pseudo-inclusions and pseudo-grooves. From a practical point
of view, however, it seems reasonable to reserve the term
"pseudo-inclusion" for the inclusions of artefacts seen in benign
lesions. (If the smears are not wet-fixed, we can observe "typical"
nuclear inclusions. The other type of artefacts may be seen in cases
where vacuolization is present, even in euthyroid nodular goiter, for
hormonal reasons.)
A peripheral
sharp dark ring of condensed chromatin is present in most of
the cases stained by the Papanicolau method . Even more important is
the presence of nuclear grooves and inclusions
within the nuclei. Both of these are artefacts,
and reflect intranuclear cytoplasmic invagination. The precise name
would be pseudo-inclusions and pseudo-grooves. From a practical point
of view, however, it seems reasonable to reserve the term
"pseudo-inclusion" for the inclusions of artefacts seen in benign
lesions. (If the smears are not wet-fixed, we can observe "typical"
nuclear inclusions. The other type of artefacts may be seen in cases
where vacuolization is present, even in euthyroid nodular goiter, for
hormonal reasons.)
(In our opinion, this is one of the most important and problematic
fields in thyroid cyt ology and also in
pathology. For this reason we deal with it
in detail in a distinct
chapter.) Briefly, we consider the presence or absence of nuclear
inclusions and grooves is to be the most important factor in the
diagnosis of papillary cancer. In some cases, the inclusions and/or
grooves are rare, but we have seen them in all smears stained by the
Papanicolau method, with only one exception. These features may also be
observed in all other carcinomas of the thyroid, a fact which is not
problematic as concerns its diagnostic power. A matter of greater
concern is the observation that a relatively large number of nuclear
inclusions and grooves may be observed in oncocytic cells derived from
Hurthle-cell adenoma or Hashimoto's thyroiditis.
ology and also in
pathology. For this reason we deal with it
in detail in a distinct
chapter.) Briefly, we consider the presence or absence of nuclear
inclusions and grooves is to be the most important factor in the
diagnosis of papillary cancer. In some cases, the inclusions and/or
grooves are rare, but we have seen them in all smears stained by the
Papanicolau method, with only one exception. These features may also be
observed in all other carcinomas of the thyroid, a fact which is not
problematic as concerns its diagnostic power. A matter of greater
concern is the observation that a relatively large number of nuclear
inclusions and grooves may be observed in oncocytic cells derived from
Hurthle-cell adenoma or Hashimoto's thyroiditis.
The occurrence of nuclear inclusions and
grooves is greatly influenced by the staining method applied. Their
occurrence in hematoxylin-eosin-stained smears is very infrequent . For this
reason, this method of staining is not accepted in thyroid cytology.
Like other nuclear details, nuclear grooves and inclusions are best
seen in Papanicolau-smears. The Wright-Giemsa method is less sensitive
than the Papanicolau method in this field. On the other hand, the
former has better specificity.
. For this
reason, this method of staining is not accepted in thyroid cytology.
Like other nuclear details, nuclear grooves and inclusions are best
seen in Papanicolau-smears. The Wright-Giemsa method is less sensitive
than the Papanicolau method in this field. On the other hand, the
former has better specificity.
Other
features
 Psammoma
bodies, which are generally regarded as a specific feature for
papillary carcinoma, are observed in about 20% of papillary carcinomas.
The classical picture of concentrically arranged, multilayered rings,
produced by changing the focus of the microscope is rarely seen. On the
other hand, benign thyroid lesions, most frequently multinodular
goiters, may also contain psammoma bodies. In the absence of cytologic
features suggestive of papillary cancer, isolated psammoma bodies are
unreliable as a predictor of papillary cancer.
Psammoma
bodies, which are generally regarded as a specific feature for
papillary carcinoma, are observed in about 20% of papillary carcinomas.
The classical picture of concentrically arranged, multilayered rings,
produced by changing the focus of the microscope is rarely seen. On the
other hand, benign thyroid lesions, most frequently multinodular
goiters, may also contain psammoma bodies. In the absence of cytologic
features suggestive of papillary cancer, isolated psammoma bodies are
unreliable as a predictor of papillary cancer.
In our practice, a definitive true psammoma body has been observed in
only 1 case of papillary cancer. In 12 other cases, we have seen dense
deposits resembling psammoma bodies. In all of these cases, other
features of the tumor were prominent. Hence, the occurrence of
(suspected) psammoma bodies was not of great help.
 The
presence of multinucleated giant cells is observed in
a majority of cases (in our practice in 57% of the cases). Kini et al.
reported a similar incidence . In most cases, the presence or absence
of multinucleated giant cells is not of great relevance. It could have
been of great help in one of our false-negative cases. This patient
also had a hyperthyroidism, treated a with thyrostatic drug. The
cytologic picture revealed a nuclear enlargement, with a variation of
shape. Neither a papillary fragment nor nuclear inclusions or grooves
could be observed in smears stained by the hematoxylin-eosin and the
Wright-Giemsa methods. One year later, we performed a repeat FNAC and
at this time we were able to give the correct cytological diagnosis
(based on the occurrence of nuclear grooves in Papanicolau-stained
smears). A reviewing of the former smears highlighted two features: a
significant number of cells were oval, and some multinucleated giant
cells could be observed. Oval cells may be seen only sporadically in
hyperthyroidism. Moreover, analysis of the smears from patients with
hyperthyroidism demonstrated that the occurrence of multinucleated
giant cells is a quite sensitive and very specific feature as concerns
the presence of papillary cancer.
The
presence of multinucleated giant cells is observed in
a majority of cases (in our practice in 57% of the cases). Kini et al.
reported a similar incidence . In most cases, the presence or absence
of multinucleated giant cells is not of great relevance. It could have
been of great help in one of our false-negative cases. This patient
also had a hyperthyroidism, treated a with thyrostatic drug. The
cytologic picture revealed a nuclear enlargement, with a variation of
shape. Neither a papillary fragment nor nuclear inclusions or grooves
could be observed in smears stained by the hematoxylin-eosin and the
Wright-Giemsa methods. One year later, we performed a repeat FNAC and
at this time we were able to give the correct cytological diagnosis
(based on the occurrence of nuclear grooves in Papanicolau-stained
smears). A reviewing of the former smears highlighted two features: a
significant number of cells were oval, and some multinucleated giant
cells could be observed. Oval cells may be seen only sporadically in
hyperthyroidism. Moreover, analysis of the smears from patients with
hyperthyroidism demonstrated that the occurrence of multinucleated
giant cells is a quite sensitive and very specific feature as concerns
the presence of papillary cancer.
Cystic degeneration. The presence of a
small number of macrophages is quite often seen in papillary cancer. If
the tumor has undergone cystic degeneration, the correct cytodiagnosis
may be very difficult or even impossible. In these cases,
non-cytological  features
may be of help. The aspirated fluid
is brown in most cases of cystic papillary cancers. In one of our
cases, metastatic, cystic papillary cancer was suspected on the basis
of the elevated thyroxine content of the cystic fluid. US-guided FNAC
has a great role in this field. After evacuation of a cyst, we perform
a repeat US at once, with US-guided FNAC on the solid remnant of the
nodule. However, in a significant proportion of cystic thyroid nodule
cases, the FNAC report remains non-diagnostic. In those cases where the
cyst recurs, surgery should be considered as a possibility.
features
may be of help. The aspirated fluid
is brown in most cases of cystic papillary cancers. In one of our
cases, metastatic, cystic papillary cancer was suspected on the basis
of the elevated thyroxine content of the cystic fluid. US-guided FNAC
has a great role in this field. After evacuation of a cyst, we perform
a repeat US at once, with US-guided FNAC on the solid remnant of the
nodule. However, in a significant proportion of cystic thyroid nodule
cases, the FNAC report remains non-diagnostic. In those cases where the
cyst recurs, surgery should be considered as a possibility.
 Focal
oncocytic metaplasia. This phenomenon is often seen, and is
mentioned by most published papers. On the other hand, it is not of
major significance, and in no way aids the diagnosis of papillary
cancer. It seems that oncocytic metaplasia (including its diffuse form,
the oxyphilic cell variant of papillary cancer) occurs somewhat, but
not
significantly more frequently in papillary cancer than in follicular
tumors or nodular goiter. The reason for this may be that degenerative
metaplastic changes occur more often in carcinoma cells as compared
with non-malignant lesions.
Focal
oncocytic metaplasia. This phenomenon is often seen, and is
mentioned by most published papers. On the other hand, it is not of
major significance, and in no way aids the diagnosis of papillary
cancer. It seems that oncocytic metaplasia (including its diffuse form,
the oxyphilic cell variant of papillary cancer) occurs somewhat, but
not
significantly more frequently in papillary cancer than in follicular
tumors or nodular goiter. The reason for this may be that degenerative
metaplastic changes occur more often in carcinoma cells as compared
with non-malignant lesions.
 Scattered
lymphocytes in smears from papillary carcinoma are a common
finding. On the other hand a large number of lymphocytes may cause a
serious differential diagnostic problem first of all if follicular
cells present oxyphilic metaplasia. The differentiation between a
Hashimoto's thyroiditis with or without papillary carcinoma may be
impossible in cert
Scattered
lymphocytes in smears from papillary carcinoma are a common
finding. On the other hand a large number of lymphocytes may cause a
serious differential diagnostic problem first of all if follicular
cells present oxyphilic metaplasia. The differentiation between a
Hashimoto's thyroiditis with or without papillary carcinoma may be
impossible in cert ain
cases.
ain
cases.
We observed squamous cell metaplasia of papillary
carcinoma cells in around 3% of our cases. Differential diagnostic
problems arose in one of our cases but the thyroglobulin determination
in the wash-out of the needle decided the issue.
The  presence
of different population of cells on the same smear
may cause severe differential diagnostic problems. Fortunately, this is
a rare situation. If we gain the cytological sample from the adequate
localization than the dominant cell type on the smear comes from the
lesion in question and relatively small amount of cells comes from the
thyroid ventral to the nodule.
presence
of different population of cells on the same smear
may cause severe differential diagnostic problems. Fortunately, this is
a rare situation. If we gain the cytological sample from the adequate
localization than the dominant cell type on the smear comes from the
lesion in question and relatively small amount of cells comes from the
thyroid ventral to the nodule.
VARIANTS OF PAPILLARY CARCINOMA
Oxyphilic
cell variant of papillary cancer
(see chapter on oxyphilic tumors)
Follicular variant of papillary carcinoma
Diagnosis of this type
of cancer is more difficult. Papillary structures are not observed, and
the dominant follicular clusters resemble those seen in follicular
adenoma. The microfollicular structure is preserved in most cases.
Colloid is present in a relatively high number of the cases of the
follicular variant of papillary cancer. In its typical form, it appears
as thick globules. These features involve a risk of a false-negative
diagnosis. However, the nuclear details (the presence of nuclear
inclusions or grooves) are of great help  . Nevertheless,
we are of the opinion that, if only the nuclear details are suggestive
of papillary cancer, then a definitive cancer diagnosis may well be
false-positive (Mesonero 1998). 2 of our 4 false-positive
diagnoses were believed to be the follicular variant of papillary
cancer, but on histopathological examination both proved to be atypical
adenoma without any signs of invasion.
. Nevertheless,
we are of the opinion that, if only the nuclear details are suggestive
of papillary cancer, then a definitive cancer diagnosis may well be
false-positive (Mesonero 1998). 2 of our 4 false-positive
diagnoses were believed to be the follicular variant of papillary
cancer, but on histopathological examination both proved to be atypical
adenoma without any signs of invasion.
Accordingly, in this field we believe that if only nuclear details are
indicative of papillary carcinoma, the correct cytological report
should be a suspicion of (the follicular variant of) papillary
cancer. With regard to the relatively poor sensitivity of
intraoperative frozen sections in diagnosing this variant of papillary
carcinoma, the surgical procedure of choice in our practice when the
intraoperative frozen section is not conclusive is lobectomy of the
thyroid (see Chapter Guidelines to surgery).
Columnar and tall cell variant of papillary cancer
These variants are
represented by aggressive behaviour and a worse prognosis than for the
classical variant of papillary carcinoma.  The tall cell
variant exhibits cells with a height/width ratio > 2, single nuclei
and an abundant basophilic cytoplasm. These features resemble those of
Hürthle-cell tumors. The characteristic cytologic features of the
columnar cell variant are similar to those of the classical variant
except for a higher number of columnar cells and the presence of a
complex microfollicular pattern. The stratification of the nuclei
covering the tissue fragments and clusters of large cells with
vacuolated cytoplasm are also among the important cytologic features
mentioned in the literature. Metastatic adenocarcinoma is the most
important disease in the differential diagnostics of the columnar cell
variant. Immunocytochemistry is not always of help, because the cells
from columnar cell carcinoma are less differentiated.
The tall cell
variant exhibits cells with a height/width ratio > 2, single nuclei
and an abundant basophilic cytoplasm. These features resemble those of
Hürthle-cell tumors. The characteristic cytologic features of the
columnar cell variant are similar to those of the classical variant
except for a higher number of columnar cells and the presence of a
complex microfollicular pattern. The stratification of the nuclei
covering the tissue fragments and clusters of large cells with
vacuolated cytoplasm are also among the important cytologic features
mentioned in the literature. Metastatic adenocarcinoma is the most
important disease in the differential diagnostics of the columnar cell
variant. Immunocytochemistry is not always of help, because the cells
from columnar cell carcinoma are less differentiated.
Cytological differential diagnostics
Nodular goiter
This type of lesion
displays hyperplastic papillae in a significant number of cases. It is
usually possible to make a clear distinction between benign and
malignant papillarization TABLE.
It is not the type of the papillary frond that is the important
feature, but the presence or absence of the nuclear atypia
characteristic of papillary cancer. However, it may be difficult to
make a clear distinction when the follicular cells are not well
preserved (cystic degeneration) or metaplastic (oxyphilic cell
alteration). If well-preserved follicular cells are not seen, or only
sporadically, a differentiation between nodular goiter and papillary
cancer may be impossible. Additional  features
may be suggestive of the
nature of the disease. The presence of colloid, and the light colour of
the aspirated cystic fluid favour a benign disease. On the other hand
the dark-brown colour of the cystic fluid is of less relevance. If even
repeat FNAC is not diagnostic, then the size of the lesion and the age
and particularly the compliance of the patient help us to decide. If
the lesion is not larger than 2 cm, and the patient is young, we can
recommend follow-up (initially 6 months, and then once a year). If the
lesion grows in size, surgery is mandatory. On the other hand, if the
uncertainty causes the patient psychological stress, and we are unable
to relieve this, then we recommend operation even for lesions as small
as 1 cm in maximal diameter. In most cases, providing the patient with
detailed information is sufficient for reassurance, and only a few of
them then decide to undergo surgery.
features
may be suggestive of the
nature of the disease. The presence of colloid, and the light colour of
the aspirated cystic fluid favour a benign disease. On the other hand
the dark-brown colour of the cystic fluid is of less relevance. If even
repeat FNAC is not diagnostic, then the size of the lesion and the age
and particularly the compliance of the patient help us to decide. If
the lesion is not larger than 2 cm, and the patient is young, we can
recommend follow-up (initially 6 months, and then once a year). If the
lesion grows in size, surgery is mandatory. On the other hand, if the
uncertainty causes the patient psychological stress, and we are unable
to relieve this, then we recommend operation even for lesions as small
as 1 cm in maximal diameter. In most cases, providing the patient with
detailed information is sufficient for reassurance, and only a few of
them then decide to undergo surgery.
Hyperthyroidism
If a patient with
Graves-Basedow's disease exhibits a nodule (which is the situation in
around 25 % of our cases), FNAC must be performed. The risk of
papillary cancer in nodules in Graves-Basedow's disease is similar to
that for a euthyroid patient. The interpretation of the cytology is
somewhat more difficult, because the atypia caused by hyperthyroidism
and/or the thyrostatic agent  may
contribute to the cytological picture.
may
contribute to the cytological picture.
It must be borne in mind that either Graves-Basedow's disease or the
thyroid nodule itself is a condition which may indicat surgery. From a
clinical aspect, if a patient with hyperthyroidism has a nodule, the
question of surgery must be assessed very carefully. If there is any
doubt about the benign nature of the disease following microscopic
analysis, surgery should be proposed.
There is another problem. In autoimmune thyroid disorders, the presence
or absence of a nodule is not always clear. Increase in the
vascularization of the thyroid, as in hyperthyroidism, causes
alterations in the palpation: the thyroid becomes firm. The thyroid is
enlarged, and this enhances the extent of small irregularities in the
ventral contours of the gland. This is one of the conditions where the
palpation may overdiagnose a thyroid nodule. Moreover, the acute phase
of hyperthyroidism is the only condition where US may fail to detect
all of the lesions. It is well known that hyperthyroidism
demonstrates either diffuse hypoechogenicity or a patchy hypoechogenic
pattern. Thyroid malignancies primarily occur in nodules with low
echogenicity.) On the other hand, US may reveal discrete
echoabnormalities which are not nodules with potentially malignant
behaviour, but rather one of the appearance forms of Graves-Basedow's
disease. Thus, if there is no doubt that a nodule is present, but doubt
arises concerning its benign nature, surgery is mandatory. If the
presence of the nodule is questionable and the cytology is not clearly
benign, we again consider the indication of surgery. To avoid a failure
to detecting papillary cancer (which occurred in one of our cases), we
perform a second US 6 months after the beginning of the disease. At
this time, the low echogenicity caused by the hyperthyroidism has
decreased, and hypoechogenic nodules missed at the first US may be
detected.
Now back to the microscope. The atypia caused by hyperthyroidism is
reflected by nuclear enlargement, and a great variability in nuclear
size. However, the cells are characteristically round. Moreover,
nuclear grooves are not present. The characteristic vacuolization
caused by hyperthyroidism makes it difficult or impossible to interpret
nuclear vacuoles. The cells are arranged in loose cohesive monolayers,
which is a clear distinction from sheets of papillary cancer. In 2 of
our patients, typical papillary fragments with nuclear details
resembling those of papillary cancer were observed. These papillary
fronds were compact structures, which we observed in the hyperthyroid
phase but neither at the second FNAC, performed in a euthyroid state,
nor on histopathological examination. We proposed going on surgery
because US revealed discrete lesions in both patients. The
histopathology demonstrates benign disease in both cases, and
interestingly the final histological report was LT in both cases. The
hyperthyroidism was hashitoxicosis.
Hashimoto's thyroiditis
The reported incidence
of Hashimoto's thyroiditis in cases of papillary cancer varies greatly
in the literature (Kashima 1998). This is connected with
the problem of terminology in the various forms of LT (Selzer
1977 , Kashima 1998). Nuclear grooves, peripheral
sharp dark rings of condensed chromatin, and of course oxyphilic cell
changes, with nuclear atypia (even pronounced) are frequently
encountered in Hashimoto's thyroiditis. There are two conditions where
diagnostic difficulties arise. The first is, when there are only a few
lymphocytes in the smear, but US reveals a characteristic picture of LT
(diffuse hypoechogenic pattern without a discrete nodule). In these
cases, US is of great help. Although Hashimoto's thyroiditis is not
infrequently associated with small foci of papillary cancer, in our
practice no papillary cancer occurred in LT without discrete lesions on
preoperative US.
More concerns arise in the second condition, when we see nuclear
features resembling papillary cancer in a discrete lesion. Four factors
must be kept in mind in the decision-making: the number of lymphocytes
in the  smear, the
size and number of the lesions, the shape of the
lesions, and the cell type in which nuclear grooves are observed. TABLE If there
are many lymphocytes in the smear, the problem is
unfortunately not resolved and some patients with a benign disease may
undergo surgery. It may be mentioned here that the characteristic US
picture of papillary cancer, i.e. a hypoechogenic nodule with fine
granules, may be observed in more than 60 % of patient with the nodular
form of Hashimoto's thyroiditis. In the last 2 years we have frequently
decided on follow-up in questionable cases. If there are no conclusive
signs of papillary cancer, we perform repeat US (and cytology) 3 and 6
months later, and than every year. If the patient accepts our
suggestion, we do not give a definitive diagnosis after the first
examination. If the patient refuses follow-up, we give a diagnosis of
suspicion of papillary cancer. TABLE
smear, the
size and number of the lesions, the shape of the
lesions, and the cell type in which nuclear grooves are observed. TABLE If there
are many lymphocytes in the smear, the problem is
unfortunately not resolved and some patients with a benign disease may
undergo surgery. It may be mentioned here that the characteristic US
picture of papillary cancer, i.e. a hypoechogenic nodule with fine
granules, may be observed in more than 60 % of patient with the nodular
form of Hashimoto's thyroiditis. In the last 2 years we have frequently
decided on follow-up in questionable cases. If there are no conclusive
signs of papillary cancer, we perform repeat US (and cytology) 3 and 6
months later, and than every year. If the patient accepts our
suggestion, we do not give a definitive diagnosis after the first
examination. If the patient refuses follow-up, we give a diagnosis of
suspicion of papillary cancer. TABLE
This type of problem in the field of thyroid cytology is not emphasized
by others. The routine use of US explains why we are faced with this
problem relatively frequently.
Follicular adenoma
From a practical point
of view follicular adenoma is the only tumor in the thyroid which must
be the clearly distinguished from papillary cancer (in all other
thyroid  tumors
surgery can not be avoided). In most cases, the
distinction is not difficult. If we apply the Papanicolau method for
staining, a clear distinction between follicular adenoma and the
follicular variant of papillary cancer is possible on the basis of the
occurrence of nuclear inclusions and grooves. These nuclear features
are very rare in follicular adenoma. On the other hand,
hematoxylin-eosin staining is very insensitive for the detection
of nuclear grooves and inclusions. The Wright-Giemsa staining
procedure is better than the hematoxylin-eosin method, but not as good
as the Papanicolau method in this field.
tumors
surgery can not be avoided). In most cases, the
distinction is not difficult. If we apply the Papanicolau method for
staining, a clear distinction between follicular adenoma and the
follicular variant of papillary cancer is possible on the basis of the
occurrence of nuclear inclusions and grooves. These nuclear features
are very rare in follicular adenoma. On the other hand,
hematoxylin-eosin staining is very insensitive for the detection
of nuclear grooves and inclusions. The Wright-Giemsa staining
procedure is better than the hematoxylin-eosin method, but not as good
as the Papanicolau method in this field.
Follicular and oxyphilic tumors other than typical follicular adenoma
The suspicion of these
tumors is an absolute indication for surgery, so a clear distinction
between these tumors and pa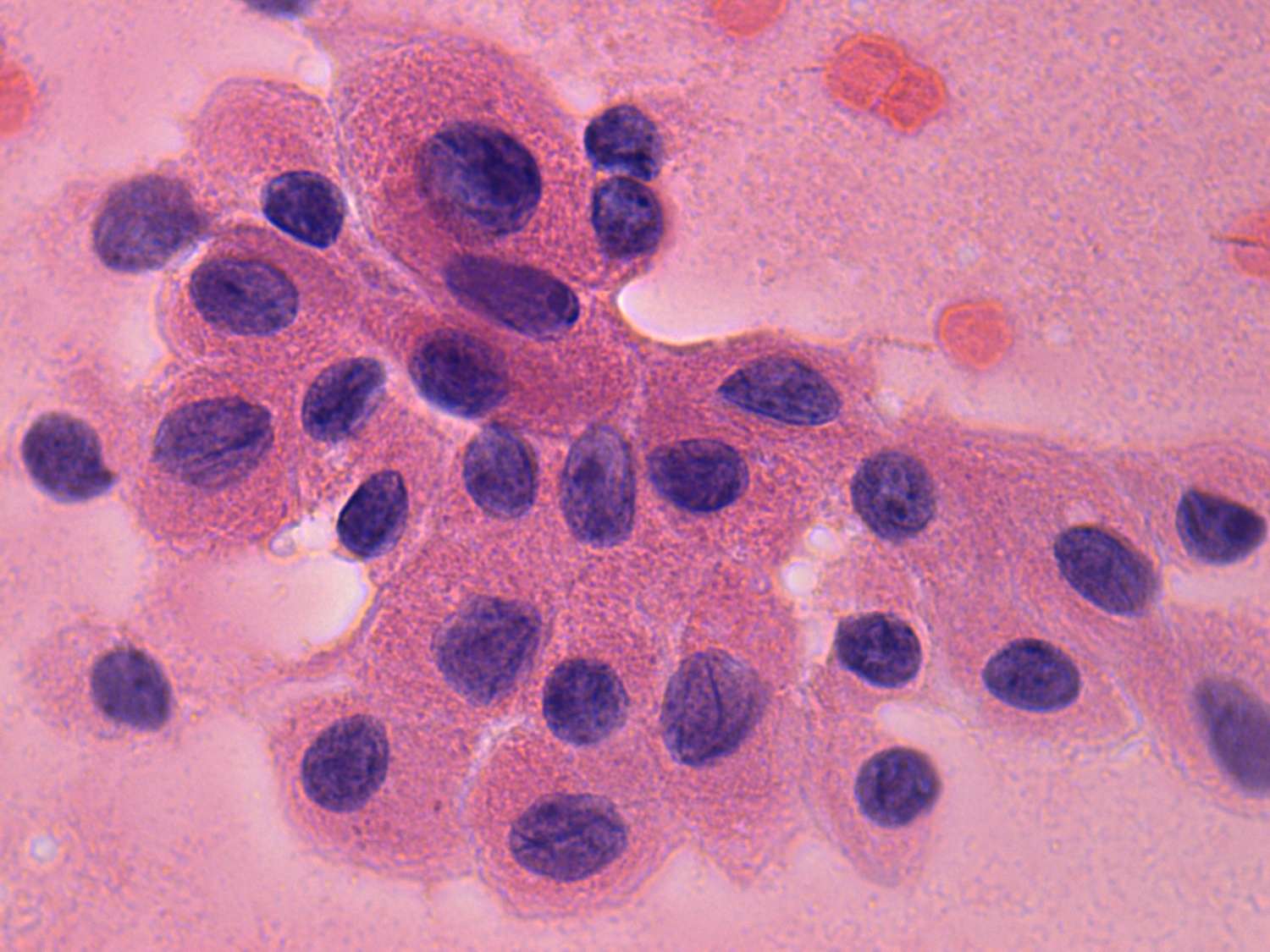 pillary
cancer seems to be of little
practical
relevance. Nevertheless, in the case of a papillary carcinoma total
thyroidectomy is the treatment of choice while to perform total
thyroidectomy in the case of a benign adenoma is a great failure.
Occasionally, these tumors may be difficult to differentiate
from papillary cancer. This is particularly true for Hürthle-cell
tumors. There may sometimes be numerous nuclear grooves in oncocytic
adenoma. The round shape of the cells, and the prominent nucleoli of
uniform size may be of great help in avoiding a false-positive
cytological report when numerous grooves
pillary
cancer seems to be of little
practical
relevance. Nevertheless, in the case of a papillary carcinoma total
thyroidectomy is the treatment of choice while to perform total
thyroidectomy in the case of a benign adenoma is a great failure.
Occasionally, these tumors may be difficult to differentiate
from papillary cancer. This is particularly true for Hürthle-cell
tumors. There may sometimes be numerous nuclear grooves in oncocytic
adenoma. The round shape of the cells, and the prominent nucleoli of
uniform size may be of great help in avoiding a false-positive
cytological report when numerous grooves are
observed. Nevertheless, a
perfect solution to this
differential-diagnostic problem can not be
achieved in all Hurthle-cell adenoma cases. A definitive cytological
diagnosis of the oxyphilic variant of papillary cancer is acceptable
only in cases where characteristic features other than nuclear
inclusions and grooves are also be observed.
are
observed. Nevertheless, a
perfect solution to this
differential-diagnostic problem can not be
achieved in all Hurthle-cell adenoma cases. A definitive cytological
diagnosis of the oxyphilic variant of papillary cancer is acceptable
only in cases where characteristic features other than nuclear
inclusions and grooves are also be observed.
